
Pharmaceutical companies operate under intense regulatory scrutiny where compliance failures carry severe consequences. Evolving regulations across regions, rising R&D costs, and complex global supply chains demand both operational efficiency and rigorous quality control.
Digital transformation offers clear benefits, but integrating new technology whilst maintaining compliance and operational continuity requires careful planning.
Emakin provides the platform flexibility pharmaceutical companies need to build automated workflows that meet their specific regulatory requirements.


Emakin provides platform capabilities that support the requirements of regulated pharmaceutical environments.
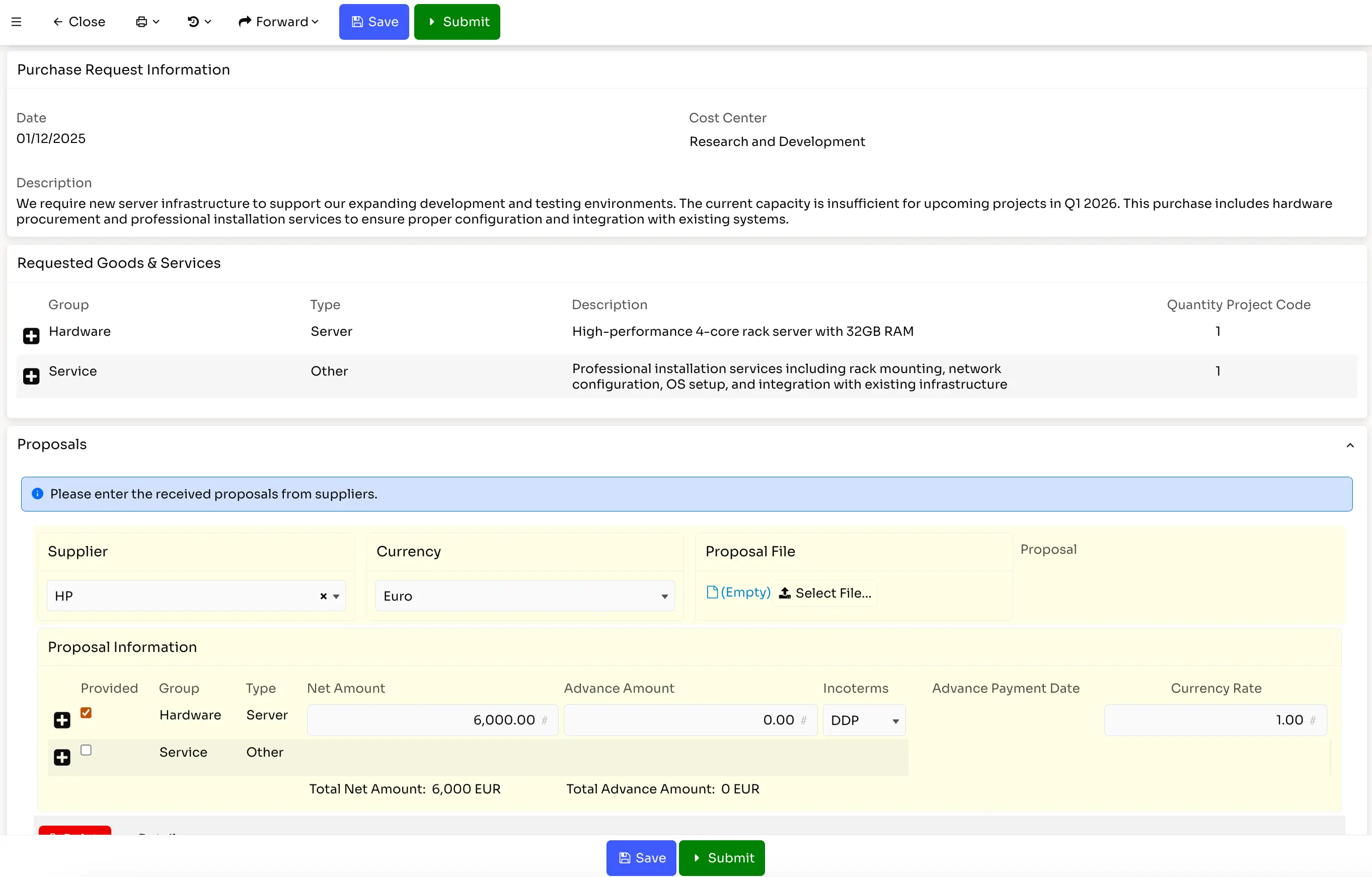
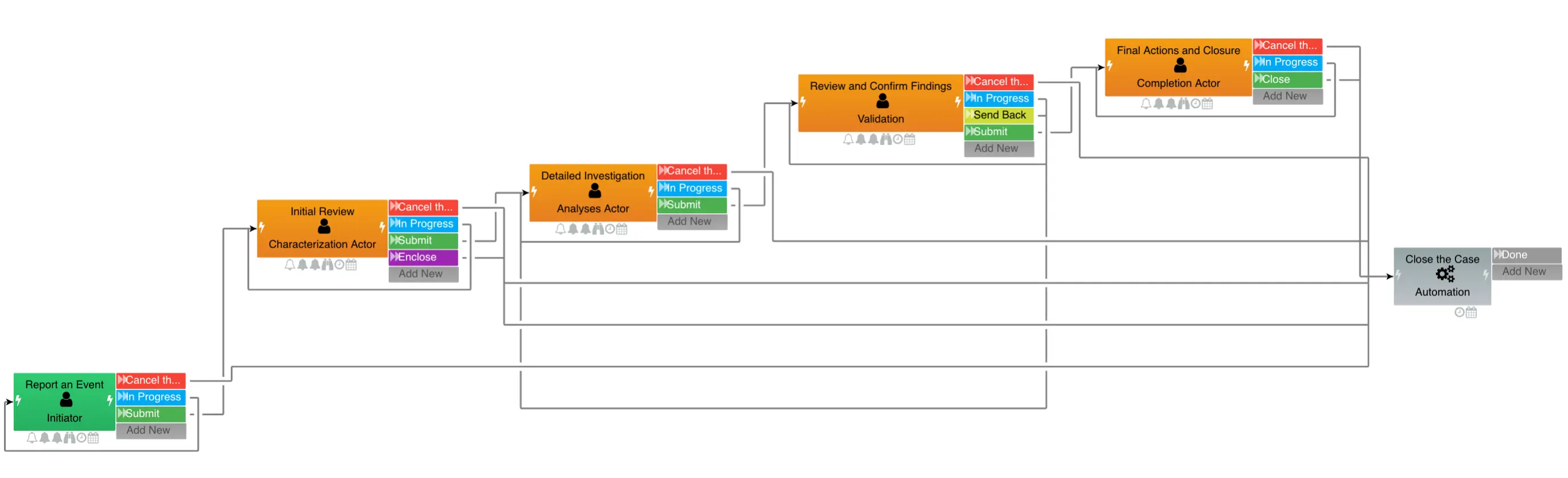
The low-code workflow designer allows you to define processes that enforce your regulatory requirements systematically. Quality procedures, trial protocols, and manufacturing processes can follow documented, auditable workflows rather than relying on email and spreadsheets.
Built-in compliance features include:

Complete audit trails capturing every action, decision, and modification

Version control and change management for process documentation

Role-based access control to enforce segregation of duties

Extensible when needed - Add custom code, scripts, and API integrations
When regulations change, workflows can be modified using visual tools without extensive redevelopment. This adaptability helps pharmaceutical companies maintain compliance as requirements evolve.







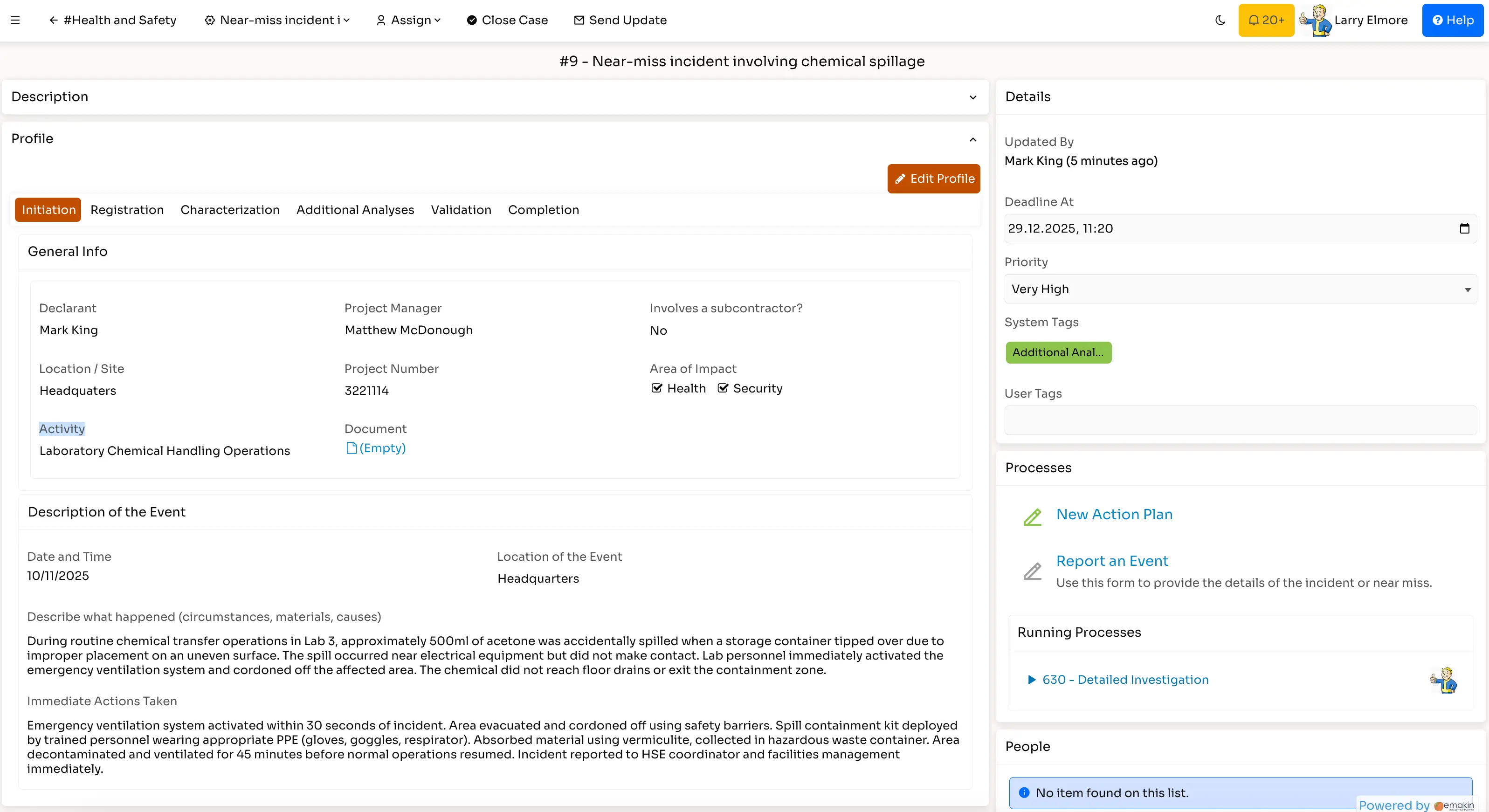
Pharmaceutical companies use Emakin to build automated workflows across various operational areas. Here are four commonly implemented process types:

Coordinate clinical trial activities, patient tracking, and protocol compliance through structured workflows. Maintain documentation trails required for regulatory submissions and ethics reviews.

Build quality management workflows for deviations, CAPA, change controls, and document management. Integrate with existing QMS systems or implement complete quality processes.

Automate order processing, batch tracking, and distribution workflows. Maintain the traceability requirements throughout your supply chain.

Streamline back-office processes like contract management, procurement, travel requests, and employee onboarding that exist in every organisation.
Pharmaceutical companies choose Emakin because the platform addresses the specific regulatory and operational requirements of the pharmaceutical sector:
Beyond the core workflows above, pharmaceutical organisations use Emakin's low-code platform to automate a wide range of processes across their operations:

Drug approval workflows

Regulatory affairs management

Intellectual property management

Managing chemical compounds

Data management and analytics

CAPA tracking

Adverse event handling

Environmental health and safety

Medical records management

Deviation management

Sales and marketing processes

Batch release processes

Partner management

Inquiry and complaint handling

Sample management

Procurement and supplier qualification

Adverse event handling

Pharmacovigilance for animal products

Veterinary medicine development

Animal health product approvals

Veterinary regulatory compliance
Everything You Need in One Platform
Your teams are ready for better ways to work. Emakin gives you the tools to build, automate, and optimise workflows quickly — so you can focus on growth, not routine tasks.